Wayne Condition College will host a scientific trial to rigorously exam for 1 yr the efficacy of a non-pharmacological medical device for Opioid Use Condition, or OUD, that could grow to be the initial therapy of its variety for the illness.
“NET System as a Non-Pharmacological Alternative to Treatment for Endorsing Opioid Abstinence,” is a U.S. Food & Drug Administration-qualifying scientific trial led by principal investigator Mark Greenwald, Ph.D., professor and Gertrude Levin Endowed Chair in Dependancy and Pain Biology in the WSU Section of Psychiatry and Behavioral Neurosciences.

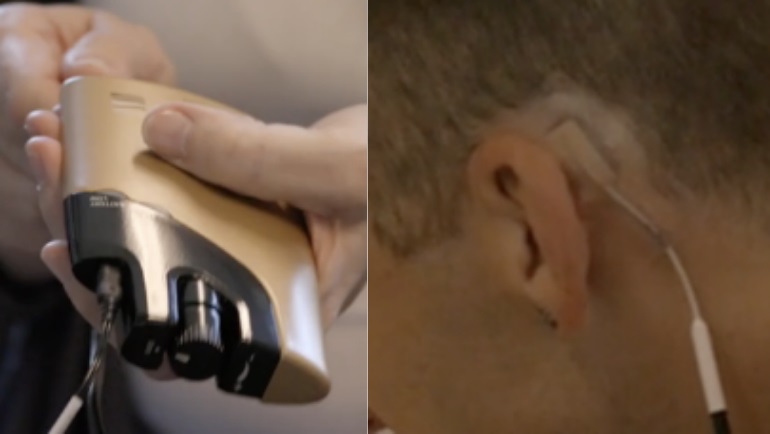
The Net, or NeuroElectric Therapy, gadget delivers alternating present-day through area electrodes placed trans-cranially on the mastoid processes, positioned at the base of the cranium on every single aspect of the head. The electrodes are connected by a cable to a regulate box. The machine provides very low-amperage waveforms at proprietary controlled frequencies and pulse widths. The individual controls the intensity of stimulation by turning a knob on the regulate box.
Net has been utilised clinically for lots of many years in Europe and South Africa, and pilot study information in Scotland and the United States has instructed it can assistance patients with OUD, Dr. Greenwald reported.
Food and drug administration-authorized Opioid Use Condition medications are helpful for quite a few, but not all clients. There is a major unmet will need for non-pharmacological OUD cure possibilities for persons who want to be opioid-abstinent with no medicine, he explained.
“Presently, OUD procedure engagement degrees are unacceptably low – only about 15{fe463f59fb70c5c01486843be1d66c13e664ed3ae921464fa884afebcc0ffe6c} of folks with OUD in the U.S. are in cure. Several men and women want helpful non-medicine cure choices – for instance, imagine of participants in 12-stage systems, which generally eschew medications – but these are presently constrained. This could guide many OUD patients to not seek out treatment method or to drop out of procedure. So, we will need to ‘meet people where they are’ to get them into remedy and remain in cure,” he included.
The Fda has said the great importance of expanding treatment method selections, especially non-opioid methods that have lessen abuse potential. Novel treatment options for OUD are not intended to replace present accepted treatment options, but as a substitute to supply safe and sound and effective possibilities to people and clinicians. The success will be introduced to the U.S. Food items and Drug Administration for a clearance final decision as quickly as they’re out there, hopefully Drop 2022, Dr. Greenwald mentioned.
“Our preliminary do the job released in early 2019 focused on analyzing the gains of Web for opioid detoxing. We identified that its efficacy for lessening opioid withdrawal and craving is comparable to Food and drug administration-approved medicines. Nonetheless, because opioid use ailment is a serious relapsing condition, we made a decision that concentrating on the brief-expression advantage of detoxing may possibly not be sufficient,” Dr. Greenwald reported. “We made a decision to deal with the far more challenging problem of looking at whether Net versus placebo stimulation in the course of the inpatient detoxing interval could have for a longer period-term efficacy on outpatient opioid abstinence. If which is the situation, that would make a large influence, and would guide to the first-of-its-sort treatment method in this condition area.”
Dr. Greenwald co-developed the medical trial with sponsor Net Restoration Corp. Main Govt Officer Joe Winston. Biostatistician and Associate Professor of Household Medicine and Public Health Sciences Samiran Ghosh, Ph.D., assisted acquire the statistical system, will randomize members to active Web as opposed to placebo, with 50 sufferers for every group (for 100 full), and will complete the statistical examination.
All info gathered for the demo – from Isaiah Household Therapy Middle, a medical therapy facility in Willisburg, Ky. – will be sent securely to an digital knowledge seize process at WSU.
Dr. Ghosh and an affiliate will be the only “unblinded” men and women. The relaxation of the investigation team will not know which patients are acquiring lively compared to placebo remedy.
“This is an unusually demanding quadruple-blinded review,” Dr. Greenwald mentioned. “We will not only measure outpatient abstinence from illicit opioid use, but will also examine irrespective of whether Web can develop these types of abstinence without having the need for Fda-permitted drugs. Secondarily, we are analyzing no matter if Web gadget use cuts down non-opioid drug use. Taken jointly, these review features hold the product to a higher standard. If effective, the machine could both be used on your own – and we would know its independent efficacy – or in mixture with other approved treatment options.”